CEFALY Enhanced is indicated to be used for:
- Acute treatment of migraine with or without aura in patients 18 years of age or older.
- Preventative treatment of episodic migraine with in patients 18 years of age or older.
CEFALY is ideal for people with migraine who:
- Don’t respond to pharmaceutical treatments or seek clinically proven effective alternatives to pharmaceuticals.
- Have prior medical conditions and are unable to take traditional pharmaceuticals.
- Have limited access to outpatient treatment procedures.
CEFALY blocks migraine pain and provides relief during attacks
In a double-blind, randomized, sham-controlled study conducted across multiple headache centers in the U.S. patients saw a reduction in migraine pain intensity following a 60-minute ACUTE treatment session with CEFALY.
- An average 59% reduction in migraine pain intensity following one hour of treatment with CEFALY. This relief continued up to 24 hours.
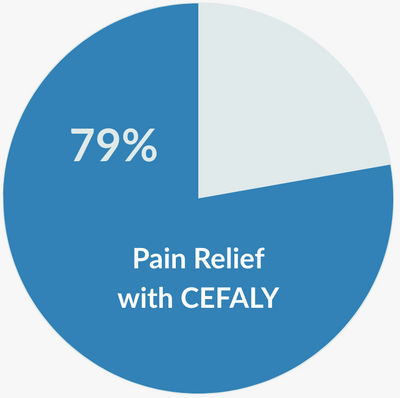
- 79% of Acute migraine sufferers saw pain relief.
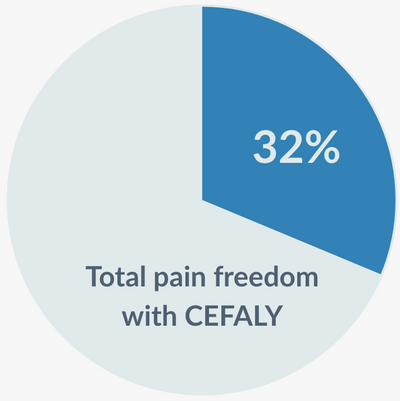
- 32% of Acute migraine sufferers saw pain freedom.
- No adverse events reported.

Read complete ACUTE Migraine Treatment Study.
Chou D. E. et al. Acute migraine therapy with external trigeminal neurostimulation (ACME): A randomized controlled trial. Cephalalgia. 2019; 39(1): 3-14.
CEFALY significantly reduces number of migraine days
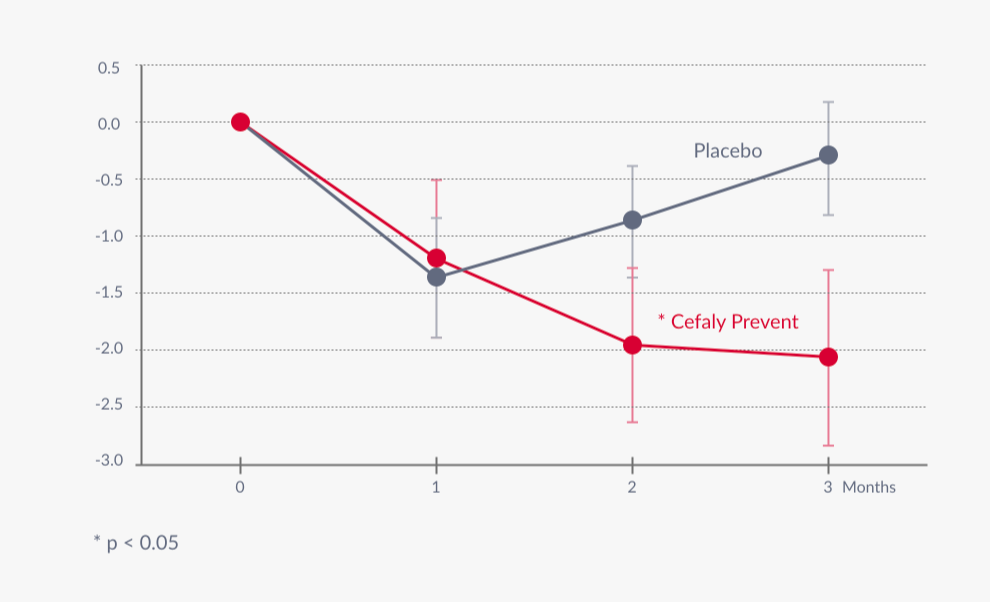
In a double-blind, randomized, sham-controlled study conducted across multiple Belgian tertiary headache centers patients with compliant daily use of CEFALY 20-minute PREVENT treatment experienced a significant reduction in number of migraine days.
- Mean 2.06 (30%) reduction in migraine days in verum group.
- 38.1% of CEFALY users saw at least 50% reduction in number of migraine days.
- Adverse events were mild and fully reversible within 20 minutes, without intervention.
Read complete PREVENT migraine study
PREMICE STUDY Migraine prevention with a supraorbital transcutaneous stimulator. A randomized controlled trial. Jean Schoenen, Bart Vandersmissen, Sandrine Jeangette, Luc Herroelen, Michel Vandenheede, Pascale Gerard, Delphine Magis. Neurology Feb 2013, 80 (8) 697-704; DOI: 10.1212/WNL.0b013e3182825055

Monthly migraine attacks, monthly headache days, and monthly acute antimigraine drug intake were significantly reduced but not in the sham group. There were no adverse events in either group.
Conclusions: CEFALY is effective and safe as a preventive therapy for migraine. The therapeutic gain (26%) is within the range of those reported for other preventive drug and nondrug antimigraine treatments.
CEFALY Enhanced has few side effects, which have been demonstrated to be minor and fully reversible with cessation of device use.
If you experience any serious side effects, or if any side effects persist for more than a few weeks, stop use of CEFALY Enhanced and consult your healthcare provider or seek immediate medical attention.
Common
- Sleepiness
- Headache After a Session
Uncommon
- Forehead Skin Redness
- Nausea
Rare
- Forehead Skin Allergy